Medical Laboratory Science (MLS) plays a vital role in the daily activities of infection prevention (IP). Though the scope is vast, one of the most important is the role MLS plays in the surveillance of healthcare associated infections (HAIs).
IPs utilize what are called “surveillance definitions” to identify HAIs, which help standardize the way IPs ultimately call different types of infections across the country. This is in contrast to what one would consider a “clinical definition,” or the clinical diagnosis of an active infection. Though the two may seem interchangeable, there are very important differences that exist between the two – most importantly that surveillance-defined events do not necessarily correlate to the existence of a true clinical infection. Inversely, not all clinical infections are going to be defined as a HAI using the respective surveillance definition. That being said, the two often exist simultaneously as surveillance definitions utilize clinical signs, symptoms, imaging, and lab results in the definition formulae.
Figure 1: Urinary Tract Infection Criteria

IPs primarily follow the National Health Safety Network (NHSN) guidelines, part of the Centers for Disease Control and a prevention (CDC), as defined in the Patient Safety Component Manual which provides standardized definitions for the surveillance of HAIs. This manual provides definitions for several event types, including Catheter Associated Urinary Tract Infections (CAUTI), Central Line Associated Blood Stream Infections (CLABSI), and Surgical Site Infections (SSI) to name a few. Additionally, NHSN provides LabID definitions for healthcare-onset Clostridioides difficile infection (CDI) and MRSA bacteremia.
For example, when defining a CAUTI, as IPs we must pull together several important elements to ultimately call an event (Figure 1).
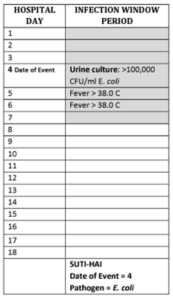
From the definition of a Symptomatic UTI (SUTI) several elements exist, including an eligible device (indwelling urinary device that has been in place for more than two (2) calendar days), a urine culture (with no more than two organisms identified, one of which must be >100k CFU), and one of several symptoms (fever, pain, tenderness, dysuria, etc.). You can see from the definition, that a defining piece of the puzzle is the results of a urine culture. In fact, this is the defining diagnostic element that sets the Infection Window Period (IWP) for the surveillance of a CAUTI – without it, there would be no event to evaluate (Figure 2).
Figure 2: SUTI Event Example
So where does the clinical lab come into play? The lab plays several important roles in this collaboration. First, the lab is responsible for evaluating the quality and appropriateness of clinical specimens. This includes validating appropriate specimen collection and test ordering, as well as proper aseptic set up of cultures. Secondly, the laboratory is vital for the appropriate interpretation of lab tests and microbial cultures. How cultures are reported can make or break the surveillance of particular infections. Lastly, the laboratory ensures the validity and quality of their results through quality assurance. Staying up to date with culture reporting guidelines (i.e., what organisms get worked up and how they get reported in the final test result) and evaluating the accuracy of testing provides an important level of assurance that the test results used in surveillance formulae are in-fact correct.
Why does this matter? Well, certain event types determined through surveillance are reported nationally to various agencies, including the Centers for Medicare and Medicaid (CMS). Through the Hospital-Acquired Condition (HAC) reduction program, CMS can withhold Medicare reimbursement from low performing hospitals in an effort to compel hospitals to focus on healthcare quality. On top of this, certain hospital safety ranking agencies, like the Leapfrog group, use NHSN-reported events in their ranking systems.
Aside from this, as mentioned before, surveillance and clinical events do not necessarily exist in a vacuum. Surveillance provides IPs with vital data that can either illuminate new opportunities or support change management of processes that will improve patient safety and health outcomes.
Sources:
National Health Safety Network (2022). Patient safety component manual. Centers for Disease Control and Prevention. https://www.cdc.gov/nhsn/index.html
